Pipeline

Nobelpharma committed to development of critical but neglected drugs in order to fulfill social mandate
We have constantly been aspiring to conduct research and development of drugs, that are not the prime targets for many pharmaceutical companies due to questionable economic viability with a small number of patients (drugs for unmet medical needs). Our development pipeline mainly includes the drugs such as orphan drugs (drugs for rare diseases) that have been strongly requested by patients, academic societies, or the government, as well as off-label drugs and pediatric medicines. The efficient research and development processes by our small but highly capable organization is what enables us to obtain approvals for such drugs with a comparatively small market size.
While we heretofore have engaged in the development of many unapproved drugs, that were already available in US and Europe but were not developed in Japan despite their significant need, we will also actively take on the challenges of research and development under new and different concepts.
Development Process of Nobelpharma
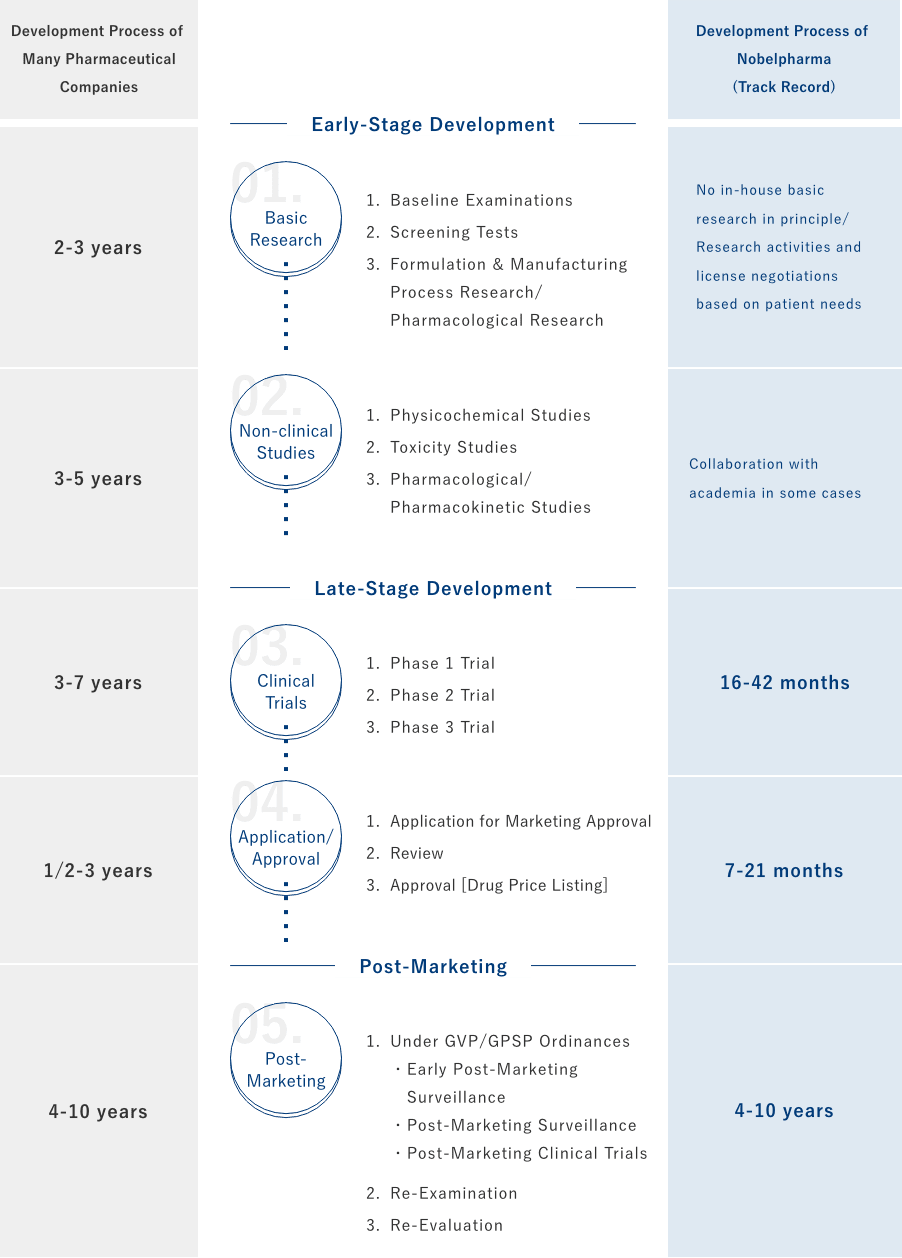
Future Development Projects
The pipeline shows the developmental status of an unapproved drug or an unapproved indication and is not intended to promote or advertise any of the drugs.
- Note: Indicagtions and Approval are merely the expectations.
New Drugs/New Devices (including global development projects)
As of January 2025
| Compound | Expected Indication |
Partner | Phase | Expected MA |
|---|---|---|---|---|
| NPC-25 zinc histizine hydrate |
hypozincemia | In-house | launched | - |
| NPC-09 aceneuramic acid |
suppression of progressive muscle weakness in GNE myopathy |
in-house | approved | - |
| NPC-26 sargramostim |
autoimmune pulmonary alveolar proteinosis | Partner Therapeutics Niigata Univ |
launched | - |
| NPC-22 scopolamine |
hypersalivation | Kitasato Univ | P-II / III in prep | Mar 2027 |
| NPC-21 anti-CMV antibody |
CMV infection | Evec | P-II | - |
| NPC-29 ubiquinol |
multiple system atrophy | Tokyo Univ | P-II | Aug 2029 |
| NPC-30 (GAIA-102) high-active NK-like cells |
neuroblastoma | GAIA BioMedicine Kyushu Univ |
P-I | - |
| NPC-31 P092 maleic acid |
prion disease | Gifu Univ | P-I in prep | - |
Life Cycle Management (including global development projects)
As of January 2025
| Compound | Expected Indication |
Partner | Phase | Expected MA |
|---|---|---|---|---|
| NPC-06 Fostoin |
neural field (new indication) |
Pfizer | P-III | Mar 2026 |
| NPC-18 RETYMPA |
ear canal regeneration (new indication) |
Kaken | P-III | Dec 2026 |
| NPC-12 RAPALIMUS |
epilepsy with focal cortical dysplasia type II (new indication) | Showa Univ | P-III | Jun 2026 |
| NPC-12 RAPALIMUS |
Pendred syndrome/DFNB4 (new indication) |
Keio Univ Kitasato Univ |
P-II | - |
| NPC-12 RAPALIMUS |
primary immunodeficiency syndrome (new indication) |
Tokyo Medical and Dental Univ National Defense Medical College |
P-II | - |
| NPC-12 RAPALIMUS |
generalized scleroderma (new indication) |
Oita Univ | P-I / II | - |
| NPC-12G RAPALIMUS Gel |
vascular abnormality-associated skin lesions (new indication) |
in-house | P-III in prep (multi-national clinical trial) |
- |
| NPC-15 Melatobel |
sleep-onset difficulty associated with mild cognitive disorder or dementia (new indication) |
in-house | P-II | - |
| NPC-15 Melatobel |
xeroderma pigmentosum (new indication) |
Kobe Univ | P-II | - |
| NPC-26 salgramostim |
non-tuberculous mycobacterial disease (new indication) |
Niigata Univ | P-II | - |
Overseas Development
As of January 2025
| Compound | Expected Indication |
Partner | Phase | Expected MA | |
|---|---|---|---|---|---|
| NPC-02 (NOBELZIN) |
Wilson’s disease | - | CH | approved | - |
| NPC-15 (MELATOBEL) |
Sleep-onset difficulty associated with neurodevelopmental disorder in children | - | CH | filed | Feb 2024 |
| NPC-17 thyroid cartilage fixation device (TITANBRIDGE) |
adductor spasmodic dysphonia | - | US | trial in prep | - |
| EU | CE marking in process | Aug 2025 | |||
| NPC-16 (JEMINA) |
dysmenorrhea | - | CH | P-III in prep | Dec 2028 |
| NPC-18 (RETYMPA) |
tympanic perforation | MEEI/ Harvard Univ/ New York Univ |
US | P-II | - |
- Research and Development
