Our Company

Pharmaceuticals and medical devices for unmet medical needs, created through our unique product planning and research and development
Factors for venture to develop new drugs one after another
Nobelpharma was founded in 2003, and in 2008 we obtained our first marketing approval for an ethical drug, NOBELZIN®. We now have marketing approvals for and sell 18 new drugs and one new medical device. In the pharmaceuticals industry, there are no companies of similar scale and age launching as many new drugs as we have. It is the pursuit of our unique product planning and research and development that has led to this achievement.
We engage in the projects of "drugs and medical devices for unmet medical needs" which are critical but neglected. We chose not to carry out the time-consuming and costly basic researches. Instead, we conduct license negotiations and research activities focusing on patient needs and select the best possible candidates (new drug candidates) from outside our company. This is because we want to deliver the drugs and medical devices for unmet medical needs to the medical front as quickly as possible, for the benefit of the patients.
Unique management strategy for speedy delivery of drugs
The starting point for all our new drug development is “patient need.” Accordingly, we prioritize the development themes that are strongly requested by patient organizations, medical institutions, and academic societies. As a result, we have surpassed some large pharmaceutical companies in achievement of gaining approvals for orphan drugs, pediatric medicines, and off-label drugs.
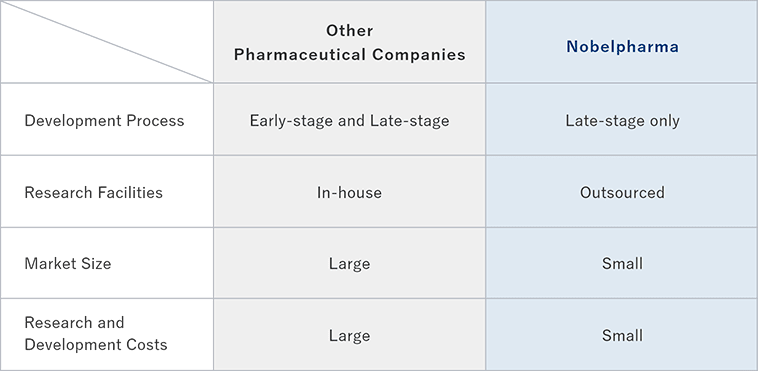
Traditional pharmaceutical companies spend five to ten years for early-stage development such as basic researches and non-clinical trials in laboratories. We employ our unique management strategy not to have laboratories and to focus on the late stage development mainly involving clinical trials. We facilitate the acceleration and optimization of our processes up to launch by taking over the early-phase development completed by academia or any other companies and advancing the development for commercialization focusing on late stage development activities of clinical trials and regulatory processes.
- Differences from Other Pharmaceutical Companies
-
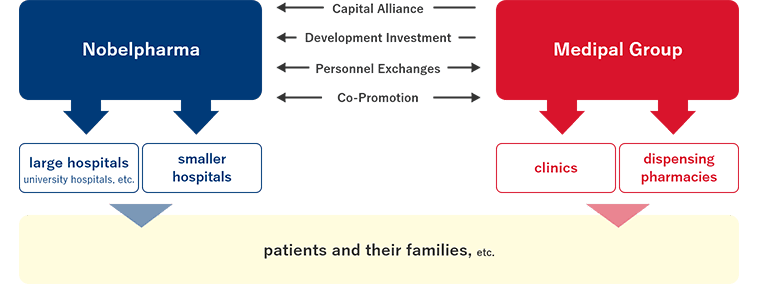
Another unique management strategy is a comprehensive partnership with MEDIPAL HOLDINGS CORPORATION for long-term and stable sales promotion activities. MEDIPAL HOLDINGS, one of the top wholesale companies in Japan, and we have been forming a mutually beneficial alliance including exclusive distribution of our products, financing by underwriting our corporate bonds, and personnel exchange especially of MRs. In 2018, MEDIPAL HOLDINGS acquired 20% of our issued shares through a third-party allocation of shares. As we became its equity-method affiliate, we have developed personnel exchange further with its group companies. We are now working even closely together to engage in educational activities for healthcare workers on rare diseases and to enhance our organization for reliable delivery of our products for unmet medical needs to patients.
- Relationship between Medipal Holdings Corporation and Nobelpharma
and Sharing of Product Promotion Activities -
Contribute to many patients through drug repositioning and lifecycle management
Nobelpharma is also focusing on "ikuyaku," drug fostering. What we think "ikuyaku" means is to find new efficacies on different diseases in our products and/or other existing drugs through drug repositioning and lifecycle management and to make them available to the patients who need them.
For example, NOBELZIN®, our first approved drug, was developed to treat the rare disease of Wilson’s disease, and its indication has been expanded through lifecycle management for hypozincemia since 2017. Likewise, the drug originally prescribed as an oral contraceptive was developed into LUNABELL® (LUNABELL® LD) for dysmenorrhea associated with endometriosis by drug repositioning and was subsequently permitted for functional dysmenorrhea by indication expansion.
Drug repositioning and lifecycle management of existing drugs enable us to reduce the time and costs for research and development, as such drugs already have the non-clinical data and have been proven for the safety through clinical trials and usage at clinical settings. We are actively engaged in lifecycle management, and we are committed to contribute to reducing distress in many patients through indication expansion of existing drugs.
Deliver new drugs to patients overseas using our expertise in new drug development
What is making possible for us to develop new drugs in a short period of time is the support and active cooperation from many physicians, medical institutions, and patients, including assemblage of required number of subjects for studies. On the other hand, we are thankfully said to be "the resort for commercialization" by some academia, possessing seeds but having difficulties commercializing. We are determined to further strengthen our cooperation with patient organizations, medical institutions, and research institutions, as well as to expand into US, China, and Europe utilizing our accumulated expertise.

